Abstract
Azacitidine (AZA) and Decitabine (DAC) are frontline hypomethylating agents (HMAs) capable of altering the natural course of myelodysplastic syndromes (MDS). Unfortunately, acquired resistance and treatment failure are hallmarks of HMA therapy. Developing therapies that effectively combine with HMAs are challenging as the underlying mechanisms driving HMA response remain uncertain. To address this, we leveraged genome-wide CRISPR-Cas9 dropout screening in a human MDS cell line in the presence of low dose AZA to identify novel synthetic lethal HMA-gene relationships.
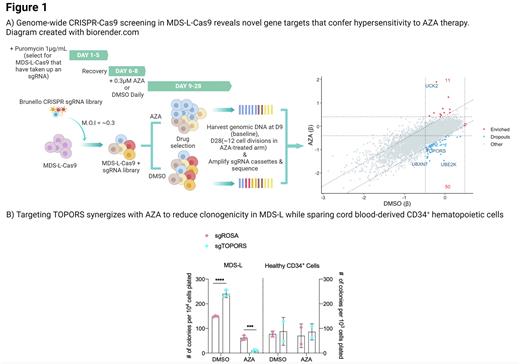
Here we identified 50 genes that, when Cas9-edited, confer hypersensitivity to AZA. These genes largely clustered into biological processes such as nucleotide excision repair and protein SUMOylation. Gene hits were ranked according to their degree of depletion under AZA selection, revealing UBXN7, UBE2K, and TOPORS as top dropout hits. In agreement with our screen, individual perturbations of UBXN7, UBE2K, or TOPORS in MDS-L significantly reduced cellular fitness under AZA selection in a competitive proliferation assay (p <.0001). Subsequent efforts focused on evaluating TOPORS as a potential target for AZA combinatorial therapy as it resulted in the strongest sensitization phenotype. We determined the dose-response relationship for AZA in TOPORS-edited MDS-L after 4 days of consecutive treatment using a range of doses. Targeting TOPORS using CRISPR-Cas9 or shRNA enhanced sensitivity to AZA by up to 3.4 fold (P<.0001). Concordantly, targeting of TOPORS synergistically reduced the clonogenicity of MDS-L exposed to AZA (P<.001). As HMAs are also used for acute myeloid leukemia (AML), we explored whether targeting TOPORS could be generalized; TOPORS-editing sensitized a panel of AML cell lines to AZA by up to 4.3-fold (P < .0001). Importantly, AZA combined with TOPORS-editing was relatively less toxic in healthy CD34+ hematopoietic cells and their function was not impaired.
TOPORS is a dual E3 ubiquitin and SUMO1 ligase implicated in regulating the DNA damage response (DDR) through SUMOylation-dependent mechanisms. Since HMAs are known to induce genome instability, we hypothesized that TOPORS contributes to genome stability in response to AZA. Apoptosis, DNA damage, and cell cycle assays revealed that TOPORS-edited MDS-L cells were primed for apoptosis as they accumulated extensive DNA damage and arrested at a late S- or G2/M-checkpoint in response to low dose AZA. Enrichment analysis of bulk transcriptomes identified activation of DNA replication and spliceosome signatures in AZA-treated TOPORS-edited MDS-L compared to control cells. According to the BioPlex interactome database, TOPORS interacts with splicing factors SRSF4 and SRSF6; therefore, we assessed the consequences of targeting TOPORS on alternative splicing. Clustering of RNA splicing profiles revealed that TOPORS-editing or AZA treatment induced global splicing alterations individually and cooperatively. Compared to AZA-treated control cells, we detected 764 misspliced events in AZA-treated TOPORS-edited MDS-L with the majority being exon skipping events (473 events, FDR<0.05, PSI>0.1). Over-representation analysis associated these misspliced genes with pathways relating to the DDR. In parallel, nuclear proteomics of AZA-treated TOPORS-edited MDS-L identified enrichment of late-stage cell cycle proteins and depletion of global nucleotide excision repair factors including SUMO1 (log2-fold change > |1|, FDR <0.05). As there are currently no pharmacological inhibitors of TOPORS, to enable clinical translation we sought to target its key biological processes. Here we piloted the combination of TAK-981, a novel high-specificity inhibitor of SUMOylation, with AZA or DAC. Combining TAK-981 and AZA was found to be cytotoxically additive in MDS-L (ZIP = 5.596 ± 1.44) and synergistic in MOLM-13 (ZIP= 11.968 ± 1.21), while TAK-981 synergized with DAC in both MDS-L (ZIP= 10.072 ± 1.21) and MOLM-13 (ZIP= 11.31 ± 3.29).
In summary, we report that targeting TOPORS confers hypersensitivity to AZA through predisposing leukemia cells to a defective DDR. The known preclinical efficacy of TAK-981 to enhance anti-tumour immune responses, combined with our findings, supports translation of HMA plus TAK-981 combinatorial therapy into early phase clinical trials for the treatment of MDS and AML.
Disclosures
Pimanda:Celgene: Research Funding; BMS: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.